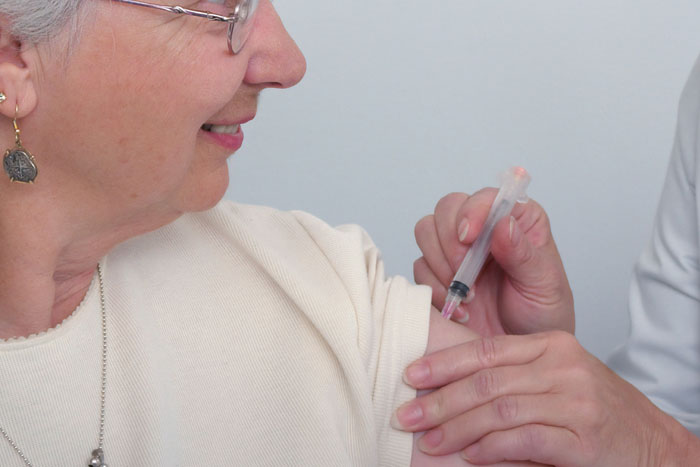
Federal health officials have revealed a contract between the present administration and two retail pharmacies, CVS and Walgreens to distribute COVID-19 vaccines to nursing homes, assisted-living facilities, and residential group homes. The contract stipulates that within 24-48 hours after the FDA approves a vaccine, trained officials of CVS and Walgreens will pay visits to interested nursing homes, assisted living facilities, and residential group homes to administer the vaccine to senior citizens who are at more risk of getting complications from coronavirus than the younger population.
Paul Mango, a senior policy adviser at the Department of Health and Human Services explained that the initiative has been established to help hard-pressed states vaccinate the members of the population with the lowest immunity to fight off the Coronavirus – the aged.
There are about 16,000 nursing homes scattered around the United States, according to the CDC, and about 20,000 – 45,000 assisted living facilities and other facilities that cater to senior citizens and the disabled. People receiving care and treatments in such facilities are less than 1% of the population but about 40% of people who have died from COVID-19 complications. According to COVID Tracking Project, more than 83,600 deaths have been recorded amongst people in these facilities.
The government had earlier tried to enforce COVID-19 testing as well as provide protective gear for members of nursing homes but the initiative was marked with a string of mistakes that led to an uproar from advocates for senior citizens and owners of nursing homes. In early October, the National Academies of Sciences, Engineering, and Medicine advised that the first round of approved vaccines should be administered to first responders and healthcare workers directly working on infected patients. The National Academies stated that elderly residents of nursing homes should then get the vaccine.
COVID-19 trials are including people who are older than 65 to answer some questions about how effectively the vaccines will protect the older population. It is also not clear how effective the vaccines will be in preventing infection. The American Health Care Association, the largest group of nursing homes in the country, commended the initiative.
“We’re recommending that all our members sign up for the program and get things ready before the FDA’s approval,” Dr. David Gifford, chief medical officer of the association announced. “Not to do that would be unfair to residents and staff.”
A smaller group that consists of nonprofit homes has shown a small reservation towards the initiative.
“It’s heartening to see that the administration’s planning has begun,” Katie Smith Sloan, president of Leading Age, said in a statement. “The vaccine is still months away, so there is time to get this right. We are happy that the administration has started plans. The vaccine won’t be available now, so we still have months to understand the initiative. We look forward to learning more about it.
The vaccination plan is one of the steps being carried out by the Operation Warp Speed, an initiative to hasten the production and distribution of hundreds of millions of doses of vaccines to Americans after the FDA approves it.
Mango stated that if any vaccine is approved before the end of the year, the first round of doses will be limited but within the first three-quarters of 2021 availability is expected to improve substantially. Officials have also announced that nursing homes and other care facilities will not pay for the vaccines. CVS and Walgreens are expected to receive the funds for the vaccines through Medicare and Medicaid.
Source: cnn.com