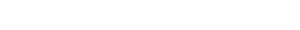Eli Lilly announced on Thursday that preliminary evidence suggests that its monoclonal antibody drug prevented COVID-19 among residents and staff of nursing homes and other assisted-living facilities in a clinical trial, in what would be the first time a drug has been shown to prevent the novel coronavirus disease.
Bamlanivimab, the Eli Lilly drug, reduced the risk of COVID-19 among staff and residents of nursing homes by a whopping 57% after doses were administered to the participants. The company said its drug was more effective among residents of such care facilities, rising to an 80% prevention rate.
The new development is welcomed, as governments around the world drive vaccination programs within their regions. The drug would provide an additional treatment option that could curb the spread of the pandemic and eradicate it in no distant time.
Lilly said it would request that US health regulators grant permission for the use of the drug across the board, especially among patients in long-term care facilities who have only recently come down with the coronavirus disease.
Lilly’s clinical trial was carried out in partnership with the National Institute of Health. It was Phase 3 or the last stage of the trial. The company said its report would be published in a medical journal, allowing for peer-to-peer review by experts.
Last year, the US Food and Drug Administration had granted permission for the use of bamlanivimab in the treatment of patients with mild to moderate form of the coronavirus disease after a previous clinical trial showed that the drug improved the health of patients.
The Chief Scientific Officer of Lilly, Daniel Skovronsky, urged the government to approve the administration of the drug as a preventive measure since not every patient, including nursing home residents, would receive COVID-19 vaccines. Skovronsky confirmed that the company’s priority right now is to see that its drug is available in all nursing homes. He confirmed that although the drug is not a replacement for vaccines, it can be useful for people yet to be vaccinated, Statnews reports.
Nursing homes have particularly been of interest to stakeholders in the fight against the virus since this population accounted for over a third of COVID-19 deaths all over the world. The nursing home residents are more susceptible to the virus for a number of reasons, including the physical condition of most facilities that don’t allow for much social distancing and the underlying health conditions suffered by the residents who are advanced in age.
The US Centers for Disease Control and Prevention said that so far, the country has administered 1.9 million COVID-19 vaccines to about 3 million residents of long-term care facilities in the US. Reports have it that staff of these facilities has been reluctant to undergo vaccination, thus slowing down vaccination efforts
For its study, which commenced in August, Lilly used 5,000 volunteers from various nursing homes who had been diagnosed with COVID-19. Lilly released the prevention data for about 965 people, with two-thirds of staff having the highest prevention rate.
The company maintains that the side effects are consistent with those as seen from previous studies, including nausea, dizziness, and headache.
The government had ordered a large shipment of the antibody from both Lilly and another drugmaker, Regeneron. Lilly collaborated in the production of its drug with AbCellera Biologics Inc., and so far, about 454,000 of the antibody has been distributed in the US.
Source: wsj.com