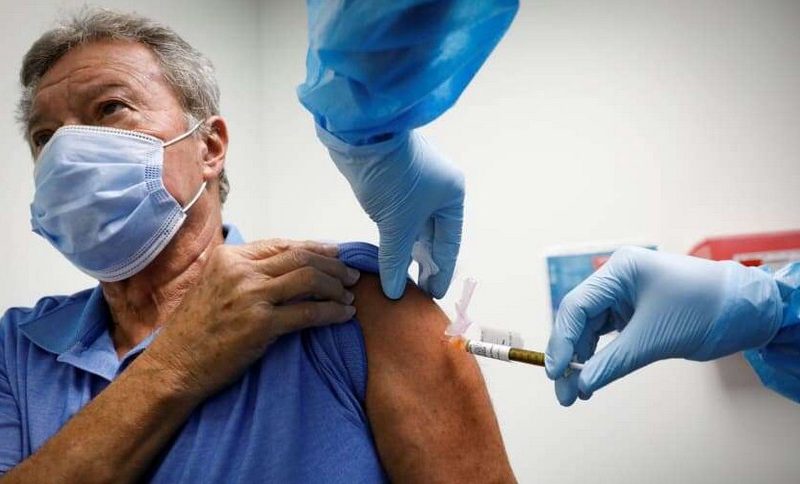
A top health official of the White House has stated that the AstraZeneca COVID-19 vaccine will not be available in the United States until April. The official explained that the administration was still skeptical about the vaccine’s effectiveness; as such, it is holding off until all questions have been satisfactorily answered, NY Times reports. The official’s statement comes a few hours after UK regulators approved the vaccine for public use on Wednesday.
The official, Moncef Slaoui, director of Operation Warp Speed, predicted earlier this month that AstraZeneca would apply for an Emergency Use Authorization within the first two months of 2021. Slaoui’s latest statements would move back the manufacturer’s timetable by about two months.
“Our current estimate, if all goes as planned, is that the AstraZeneca vaccine would be approved within the first two weeks in April,” Slaoui told reporters.
AstraZeneca vaccine trials have been fraught with different controversies including a pause during a late-stage trial as a result of widespread adverse participant reaction to the shots. There was also an episode with a concentration mistake that changed the dosage and effectiveness of the vaccine. As a result of these inconsistencies, Slaoui explained that the 300 million doses that the United States ordered will not be delivered until April.
Slaoui stated that trial results have shown that the AstraZeneca vaccine is effective in controlling severe diseases, but its effectiveness in protecting older people against the coronavirus is still unknown. This is because the trial had involved only a few old people. Slaoui stated that the vaccine’s efficacy in the protection of older people is his major concern about the adoption of the vaccine in the United States, Politico writes.
On Wednesday, British regulators approved the vaccine for public use, becoming the first country to do so. British Officials announced that the first dose of the vaccine will be administered widely, regardless of the number of people that come back for the second dose. The country plans to start administration of second doses within three months after the first doses start rolling out.
This is to ensure that many people are protected, at least to an extent, especially from the mutated strain of the virus which is much more contagious than the first strain, according to experts. But experts are still uncertain about the vaccine’s efficacy especially when there is a considerable interval between the administrations of the two doses. When a second dose is received within four weeks after the first, AstraZeneca is about 62% effective, according to officials.
The result is contrasted by the results of another AstraZeneca trial that showed 90% efficacy with only a single dose. AstraZeneca combined the results of the contrasting trials to achieve 70.4% effectiveness, a move that US health experts have denounced for being inconsistent.
Slaoui also addressed the slow distribution of the vaccine and the lukewarm response it has received. The director stated that as of Wednesday, officials of Operation Warp Speed have sent out over 14 million doses of the Moderna and Pfizer-BioNTech vaccines across the country but only 2.6 million people have received the vaccine, as of Wednesday, according to the Center for Disease Control and Prevention.
Source: nytimes.com